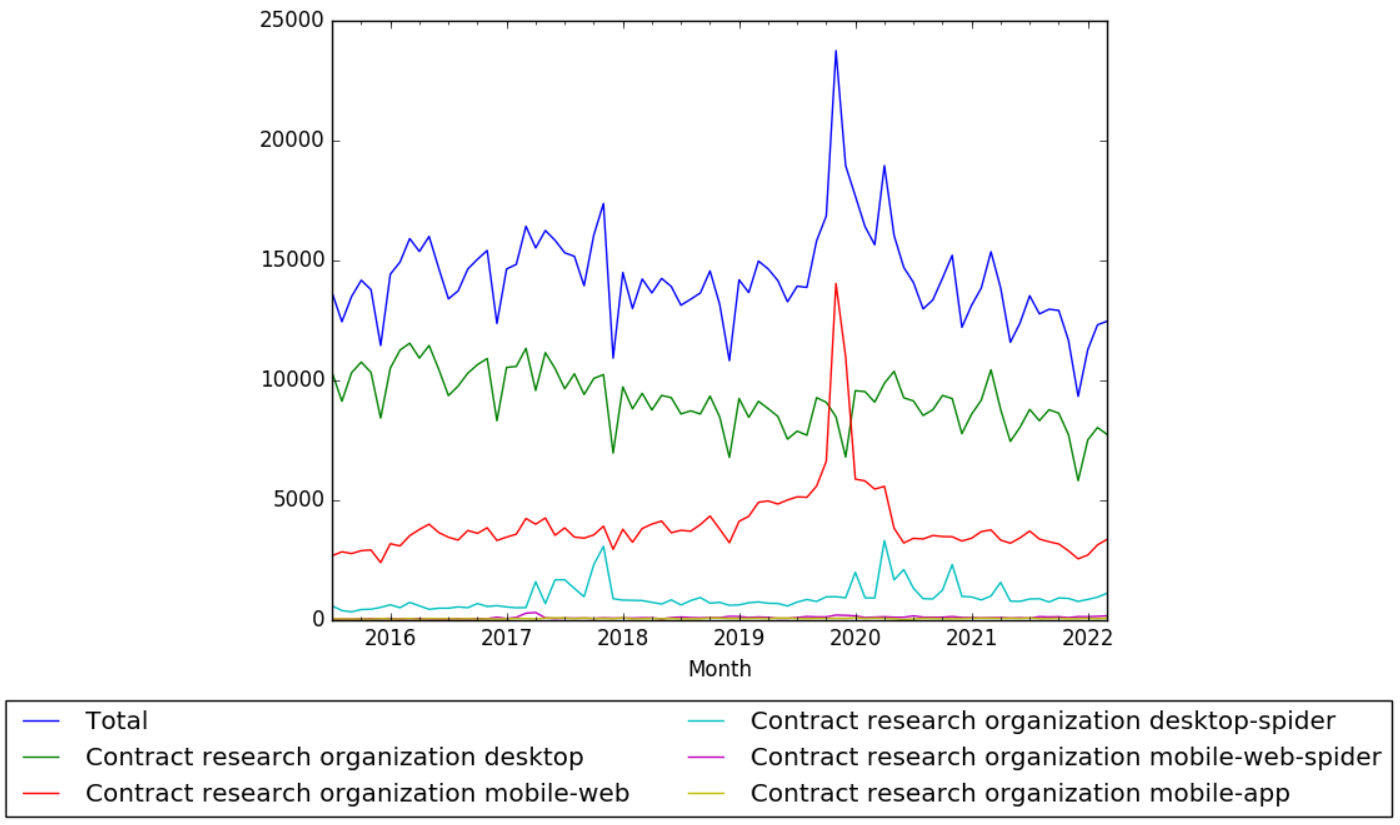
Introduction to Good Clinical Practice (GCP) Guidelines: Ensuring Quality in Clinical Research | PPT

Cyntegrity | Data Science for Clinical Trials on LinkedIn: Critical to Quality Factors in Clinical Research - ICH GCP E6 and E8
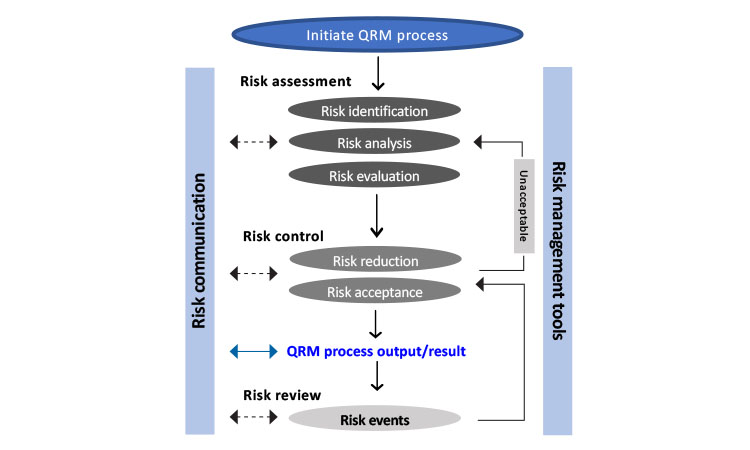
Clinical Trial Management Adaptation to ICH E6 (R2): Good Clinical Practice | Pharmaceutical Engineering